Reviewed by Sahil Chopra MD and Stacey Gunn MD.
Research by Savit Malhotra.
This article marks the fifth installment of our insomnia series. Previously, we discussed:
- What insomnia is, common causes, and potential treatment options
- The safest medications to improve sleep
- Zolpidem (Ambien)
- Trazodone and whether or not it is actually safe, and who should take the medication
This week, we will be continuing our discussion of medications available to treat insomnia. Specifically, we will be looking at the new medications that are available to treat the condition.
Introduction
The healthcare industry, especially the field of sleep medicine, is rapidly evolving, and it seems as though there is a new development every week. Previously, we have talked about many of the existing medications that people use in order to help them sleep better. This week, we’ll be taking a look at some of the newer medications available, how effective these medications are, and their safety regulations.
What Medications Already Exist
Before we begin, we’ll go through a quick breakdown of the types of medications available and some common medications that are used to treat insomnia. Insomnia medications tend to fall into one of the following categories: melatonin receptor agonists (medications that increase or promote stimulating the melatonin receptor), orexin receptor antagonists (promoting sleepiness by inhibiting wake promoting neurons), antidepressants (off-label use), antihistamines, melatonin supplements/herbal remedies, benzodiazepines, and non-benzodiazepine hypnotics (Z-Drugs). These medications tend to range from more variable efficacy but a stronger safety record to most effective and greater potential for adverse effects, with benzodiazepines and Z-Drugs being some of the riskier options.
In the previous two articles, we focused heavily on Ambien and Trazodone, a Z-Drug and an antidepressant. While these two medications are among the most commonly used, there are other medications available that have similar efficacy. For example, melatonin is another widely used sleep supplement that can be purchased over the counter and is generally considered safe for short-term use with minimal risk of dependency or tolerance.[1] In terms of melatonin’s efficacy, the medication was shown to significantly improve sleep quality while shortening sleep latency and reducing fatigue.[2] However, this was a specific case study done on those suffering from jet lag. Not all forms of insomnia result from jet lag, and in those cases, a more aggressive medication may be needed.
Dual Orexin Receptor Antagonist (DORA)
In this article, we will be looking specifically at a class of medications called dual orexin receptor antagonists, or DORAs for short. DORAs work similarly to orexin receptor antagonists, a group we previously discussed in our medications article. Dual orexin receptor antagonists work by blocking OX1R and OX2R, two receptors that the neurotransmitter orexin binds to.[13] As you’ll recall from our previous article, orexin is important in both promoting wakefulness and arousal. Thus, by blocking orexin binding, the brain’s arousal signals decrease, allowing for a better transition into sleep. Essentially, these medications work by turning the brain’s “wake switch” off.
Interestingly, orexin is also the key neurotransmitter involved in narcolepsy, a condition where a person experiences chronic sleepiness and uncontrollable sleepiness during the day.[21] Studies have found that those who have narcolepsy often do have lower orexin levels than those who don’t.[22] So in a sense, by blocking orexin levels, DORAs are able to create a state of temporary narcolepsy in the brain, promoting the onset and maintenance of sleep. This mechanism of creating short-term narcolepsy in the brain is highly effective, but it could also be the reason for some of the potential side effects (i.e. sleep paralysis, abnormal dreams, and complex sleep behaviors). We will do a more in depth analysis of the side effects later on in this article. On the note of adverse effects, it is important to note that DORAs should be prescribed with care due to their potential for side effects. A sleep medicine specialist should always be the first line of contact when it comes to medications, because everyone’s tolerance to these medications is different and other underlying conditions can lead to different effects.
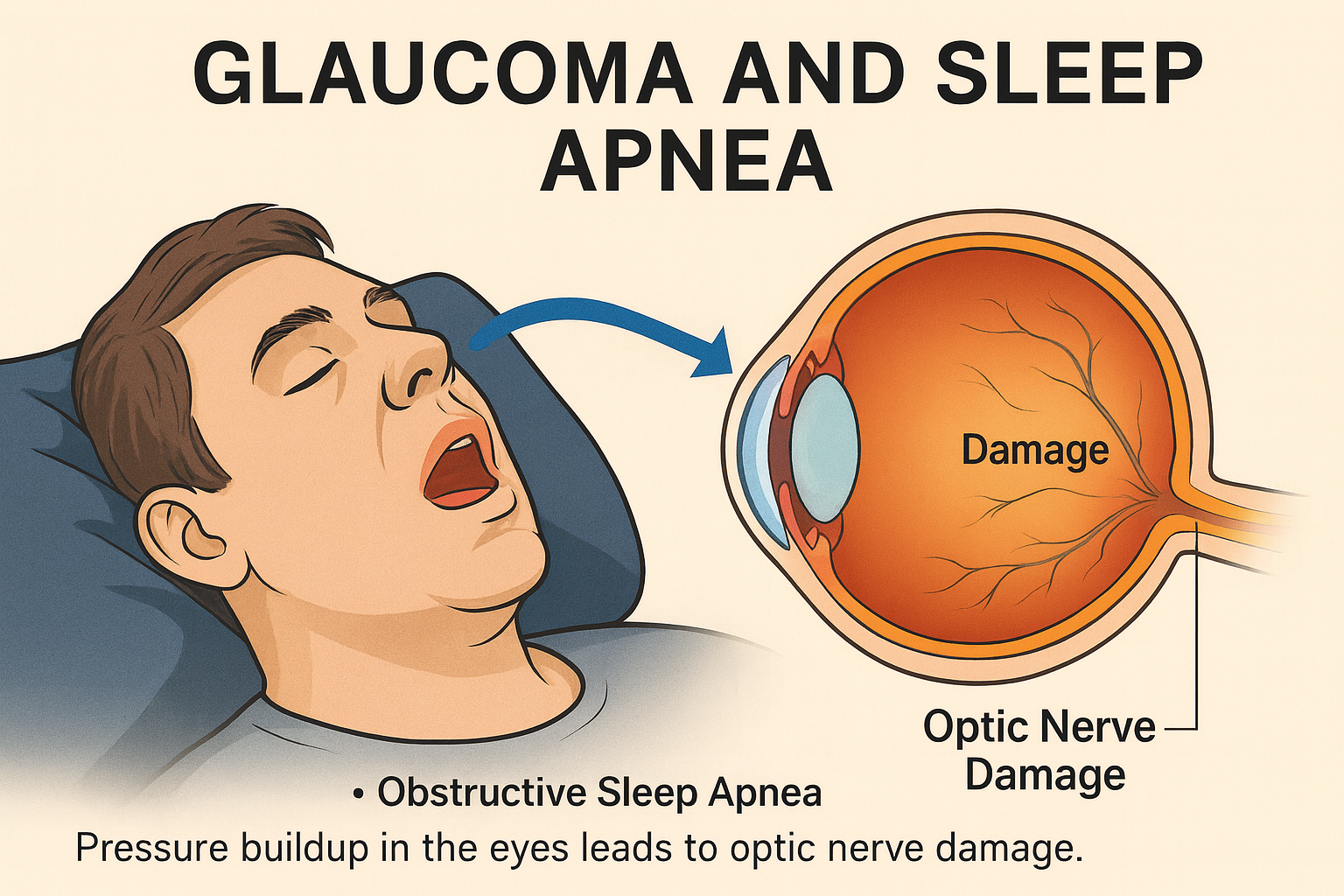
DORAs are also revolutionizing research in older adults, specifically those with Alzheimer’s disease. It is not uncommon to see patients with Alzheimer’s disease suffer from fragmented sleep and circadian rhythm disruptions.[23] Current research indicates that poor sleep/lack of sleep is associated with Alzheimer’s disease, although whether it is the poor sleep that heightens risk for Alzheimer's disease, or the underlying Alzheimer’s disease process that leads to poor sleep, is less clear.[24] The problem with other insomnia medications, such as benzodiazepines and antihistamines, is that they often lead to an increased risk of falls in these patients and can cause a morning “hangover” effect (a feeling of cognitive impairment).[25]
In recent times, DORAs have been suggested to possibly be a safer alternative. It seems as though the mechanism behind DORAs leads to them not affecting many of the motor-impairing areas of the brain.[27] These studies have also shown that, for patients with Alzheimer’s, DORAs are able to improve sleep without creating an increased risk of falls, and with less of a morning “hangover” effect.[28] Even more interesting is that some studies have drawn evidence that could suggest that DORAs may be effective in preventing the pathology of Alzheimer’s disease.For example, in animal models, there is some evidence that DORAs can decrease levels of amyloid beta and phosphorylated tau, which are proteins that build up in the brains of people with Alzheimer’s.[26] There are many ongoing studies on this topic and more data and evidence will be needed before coming to any definitive conclusion.

The image above depicts the possible mechanism/linkage between sleep deprivation and Alzheimer’s disease. All credit belongs to the original owners. Citation: Musiek, E., Xiong, D. & Holtzman, D. Sleep, circadian rhythms, and the pathogenesis of Alzheimer Disease. Exp Mol Med 47, e148 (2015). https://doi.org/10.1038/emm.2014.121
Quviviq (Daridorexant)
Now, we will begin to dive into some of the newer medications available. The first medication that we will discuss is Quviviq. Quviviq, or daridorexant, was first FDA approved on January 10th of 2022.[6]
In terms of Quviviq’s efficacy, results have shown that this medication is effective in the treatment of insomnia. One of the clinical studies of the medication found that when patients were given the medication, total sleep time was significantly improved by 22 minutes after the first month and 19 minutes after the third month. Compared with the placebo group, these findings were proven to be clinically significant.[8]
However, with any medication, there are always adverse effects that need to be noted. Quiviviq is reported to impair daytime wakefulness in individuals and can cause sleep paralysis. Sleep walking, driving, and talking were also reported to be adverse effects of the medication. In more than 5% of patients who took the medication, the most common adverse effects were reported to be headache and fatigue. As one can see, this medication shares many similar adverse effects as some of the other medications that we have previously discussed. Thus, it is important once again that those who want to take the medication should first be seen by a sleep expert to decide if the medication is actually right for them.
Dayvigo (Lemborexant)
The next medication recently approved for the treatment of insomnia is Dayvigo (lemborexant), which gained FDA approval on December 23, 2019. Like Quviviq, Dayvigo is classified as a dual orexin receptor antagonist (DORA), working by blocking the action of orexins—neurochemicals responsible for promoting wakefulness. While their mechanisms are similar, Dayvigo tends to offer a longer duration of action, helping patients stay asleep longer through the night, whereas Quviviq is noted for having a faster onset, helping users fall asleep more quickly.[10] Structurally, the two drugs differ, but these chemical differences do not majorly affect their function.
In terms of efficacy, clinical studies have shown that Dayvigo significantly improves sleep efficiency, reduces sleep onset latency, and decreases subjective wake after sleep onset compared to placebo.[11] These results suggest that Dayvigo can be effective for individuals who have trouble both falling asleep and staying asleep throughout the night.
Dayvigo's adverse effect profile is similar to that of Quviviq, with drowsiness and sleep paralysis being the most commonly reported side effects. Dayvigo has also been associated with reports of nightmares and abnormal dreams. In a study of 550 patients, 7.65% experienced drowsiness, 0.20% experienced sleep paralysis, 1.76% reported nightmares, and 0.59% experienced abnormal dreams.[12] While these effects were relatively rare, they are important considerations when selecting the best treatment option for each patient.
Belsomra (Suvorexant)
Belsomra, also known by its generic name suvorexant, is the last of the DORA-type medications that we will be talking about. Belsomra was the first of the DORA medications to be released to the public markets, gaining FDA approval in 2014.[20] Being one of the first DORA medications, Belsomra pioneered the drug market and led to many opportunities for more DORA medications to be released, like Dayvigo and Quviviq.
Although Belsomra isn’t a new medication, its efficacy still stands. One study found that those who took the medication saw a decrease in the sleep latency from 65-69 minutes to 32-35 minutes. Additionally, total sleep time saw an increase of 39 to 43 minutes per night. As one can see, the objective data shows a drastic improvement in sleep. Additionally, the subjective data aligned closely. Those who were taking the medication reported having improved sleep and decreased insomnia in comparison to the placebo group.[21]
Belsomra, like every other medication, doesn’t come without risks. The more general and common side effects that can occur while taking Belsomra include drowsiness, dizziness, headache, dry mouth, and unusual dreams.[22] However, Belsomra can also have some more severe side effects. These include worsening depression or suicidal thoughts/actions, sleep walking/talking/driving (complex sleep behaviors), sleep paralysis, and leg weakness.[23] These side effects should be kept in mind, and if any become significantly debilitating, one should consult their doctor to see if Belsomra remains a good option for them.
Conclusion
With the medical field rapidly expanding and evolving, it's no surprise that we see new medications being offered frequently. Although these medications are carefully tested and regulated, it is still important to do your own research into them and meet with professionals to decide if these medications are right for you. Here at Empower Sleep, we are always cautious when it comes to prescribing medications and want to help you choose the right treatment option. Before choosing to go the medicinal route, it is important to understand what the underlying cause of your insomnia is. Medications are best suited for short-term use, and a longer-term solution may be something like cognitive behavioral therapy. In the end, it is important to explore all available options, but even more important to understand what is causing your insomnia. This will aid in making the right choice when it comes to treatment.
References
- “8 BELSOMRA Side Effects: Common and Serious Symptoms.” GoodRx, GoodRx, www.goodrx.com/belsomra/belsomra-side-effects. Accessed 7 May 2025.
- “Belsomra (Suvorexant) FDA Approval History.” Drugs.Com, www.drugs.com/history/belsomra.html. Accessed 7 May 2025.
- “BELSOMRA® (Suvorexant) C-IV: Official Patient Website.” Belsomra, 16 Dec. 2024, www.belsomra.com/.
- Bennett, Tiffany, et al. “Suvorexant, a Dual Orexin Receptor Antagonist for the Management of Insomnia.” P & T : A Peer-Reviewed Journal for Formulary Management, U.S. National Library of Medicine, Apr. 2014, pmc.ncbi.nlm.nih.gov/articles/PMC3989084/.
- Brent A. Bauer, M.D. “Pros and Cons of Melatonin.” Mayo Clinic, Mayo Foundation for Medical Education and Research, 28 Oct. 2022, www.mayoclinic.org/healthy-lifestyle/adult-health/expert-answers/melatonin-side-effects/faq-20057874#:~:text=Melatonin%20is%20generally%20safe%20for,or%20experience%20a%20hangover%20effect.
- Celmer, Lynn. “Idorsia Receives FDA Approval of QUVIVIQ for Insomnia.” American Academy of Sleep Medicine – Association for Sleep Clinicians and Researchers, 25 Sept. 2024, aasm.org/idorsia-receives-fda-approval-of-quviviq-for-insomnia/#:~:text=On%20Jan.,onset%20and%2For%20sleep%20maintenance.
- Costello, Rebecca B, et al. “The Effectiveness of Melatonin for Promoting Healthy Sleep: A Rapid Evidence Assessment of the Literature.” Nutrition Journal, U.S. National Library of Medicine, 7 Nov. 2014, pmc.ncbi.nlm.nih.gov/articles/PMC4273450/.
- Gauld, Andrea R. “Suvorexant (Belsomra) for Insomnia.” American Family Physician, American Academy of Family Physicians, 15 June 2016, www.aafp.org/pubs/afp/issues/2016/0615/p1016.html.
- Kärppä, Mikko, et al. “Long-Term Efficacy and Tolerability of Lemborexant Compared with Placebo in Adults with Insomnia Disorder: Results from the Phase 3 Randomized Clinical Trial Sunrise 2.” Sleep, U.S. National Library of Medicine, 14 Sept. 2020, pmc.ncbi.nlm.nih.gov/articles/PMC7487867/#:~:text=Decreases%20from%20baseline%20in%20patient,serious%20TEAEs%20and%20no%20deaths.
- Lucey, Brendan P, and Erik S. Musiek. “Potential of Dual Orexin Receptor Antagonists to Prevent Alzheimer’s Disease.” Alzheimer’s & Dementia, John Wiley and Sons Inc., 3 Jan. 2025, pmc.ncbi.nlm.nih.gov/articles/PMC11710307/#:~:text=Based%20on%20its%20role%20in,onset%20of%20symptomatic%20Alzheimer’s%20disease.
- M;, Mishima K;Fujimoto K;Endo A;Ishii. “Safety and Efficacy of Lemborexant in Insomnia Patients: Results of a Postmarketing Observational Study of Dayvigo® Tablets.” Drugs in R&D, U.S. National Library of Medicine, pubmed.ncbi.nlm.nih.gov/38839690/. Accessed 7 May 2025.
- Marshall, Helen. “Quviviq (Daridorexant): Side Effects, Uses, Cost, and More.” Healthline, Healthline Media, 20 Feb. 2025, www.healthline.com/health/drugs/quviviq#faq.
- Mignot, Emmanuel, and Al. Et. The Lancet Neurology, May 2025, Volume 24, Issue 5, Pages 371-470, E8, Feb. 2022, www.thelancet.com/journals/laneur/article/PIIS1474-4422(14)70154-1/abstract.
- Musiek, Erik S, et al. “Sleep, Circadian Rhythms, and the Pathogenesis of Alzheimer Disease.” Nature News, Nature Publishing Group, 13 Mar. 2015, www.nature.com/articles/emm2014121#:~:text=AD%20patients%20often%20exhibit%20disrupted,therapeutic%20implications%20of%20these%20findings.
- “Overview: Narcolepsy.” NHS Choices, NHS, www.nhs.uk/conditions/narcolepsy/#:~:text=Narcolepsy%20is%20a%20rare%20long,orexin)%2C%20which%20regulates%20wakefulness. Accessed 7 May 2025.
- Ragsdale, S. M., et al. “Dual Orexin Receptor Antagonists as Promising Therapeutics for Alzheimer’s Disease.” Nature News, Nature Publishing Group, 8 Mar. 2025, www.nature.com/articles/s44323-025-00025-5.
- Rochon, Paula A, et al. “The Harms of Benzodiazepines for Patients with Dementia.” CMAJ : Canadian Medical Association Journal = Journal de l’Association Medicale Canadienne, U.S. National Library of Medicine, 10 Apr. 2017, pmc.ncbi.nlm.nih.gov/articles/PMC5386844/.
- Roland, Joshua P, and Donald L Bliwise. “Impact of Pharmacotherapy on Insomnia in Patients with Alzheimer’s Disease.” Drugs & Aging, U.S. National Library of Medicine, Nov. 2021, pmc.ncbi.nlm.nih.gov/articles/PMC8593056/.
- “The Science of Narcolepsy.” Sleep Medicine, sleep.hms.harvard.edu/education-training/public-education/sleep-and-health-education-program/sleep-health-education-4#:~:text=Recent%20research%20has%20revealed%20that,occurring%20at%20the%20wrong%20times. Accessed 7 May 2025.
- Wang S;Zheng X;Huang J;Liu J;Li C;Shang H; “Sleep Characteristics and Risk of Alzheimer’s Disease: A Systematic Review and Meta-Analysis of Longitudinal Studies.” Journal of Neurology, U.S. National Library of Medicine, pubmed.ncbi.nlm.nih.gov/38656621/. Accessed 7 May 2025.
























































































%20thumbnail.jpg)
.png)